Vaccine mRNA, Plasmid DNA & Spike Protein Can Persist in Humans More Than 3.5 Years After COVID Vaccination
Extremely concerning!
This article originally appeared on Focal Points and was republished with permission.
Guest post by Nicolas Hulscher, MPH
We report the longest documented persistence of mRNA vaccine components to date, independently confirmed across multiple laboratories, biospecimens, and time points using diverse analytical methods.
For years, the public was told that mRNA vaccine materials would degrade within days to weeks — rapidly broken down, biologically transient, and incapable of long-term persistence. That assumption shaped regulatory assurances, public messaging, and safety expectations worldwide. Billions across the globe received these injections based on the claim that the genetic material would quickly disappear from the body.
Today, that narrative collapses — following a coordinated, multi-country investigative effort involving the McCullough Foundation, the INMODIA laboratory (Germany), the Municipal Hospital Dresden-Friedrichstadt (Germany), Neo7Bioscience, and collaborating independent laboratories.
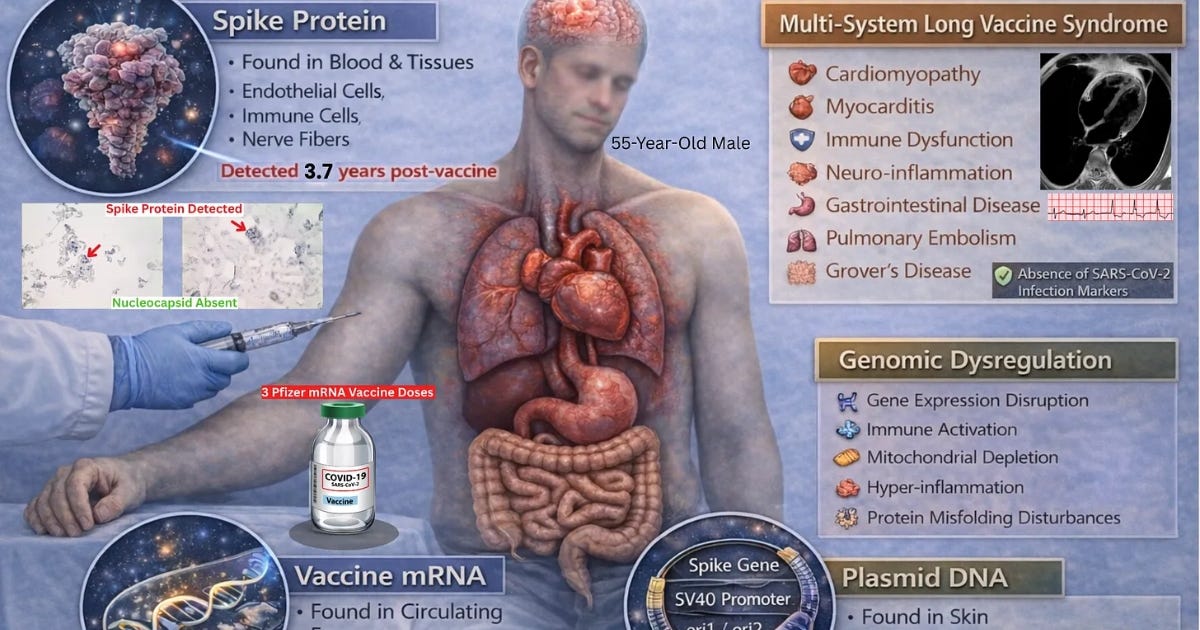
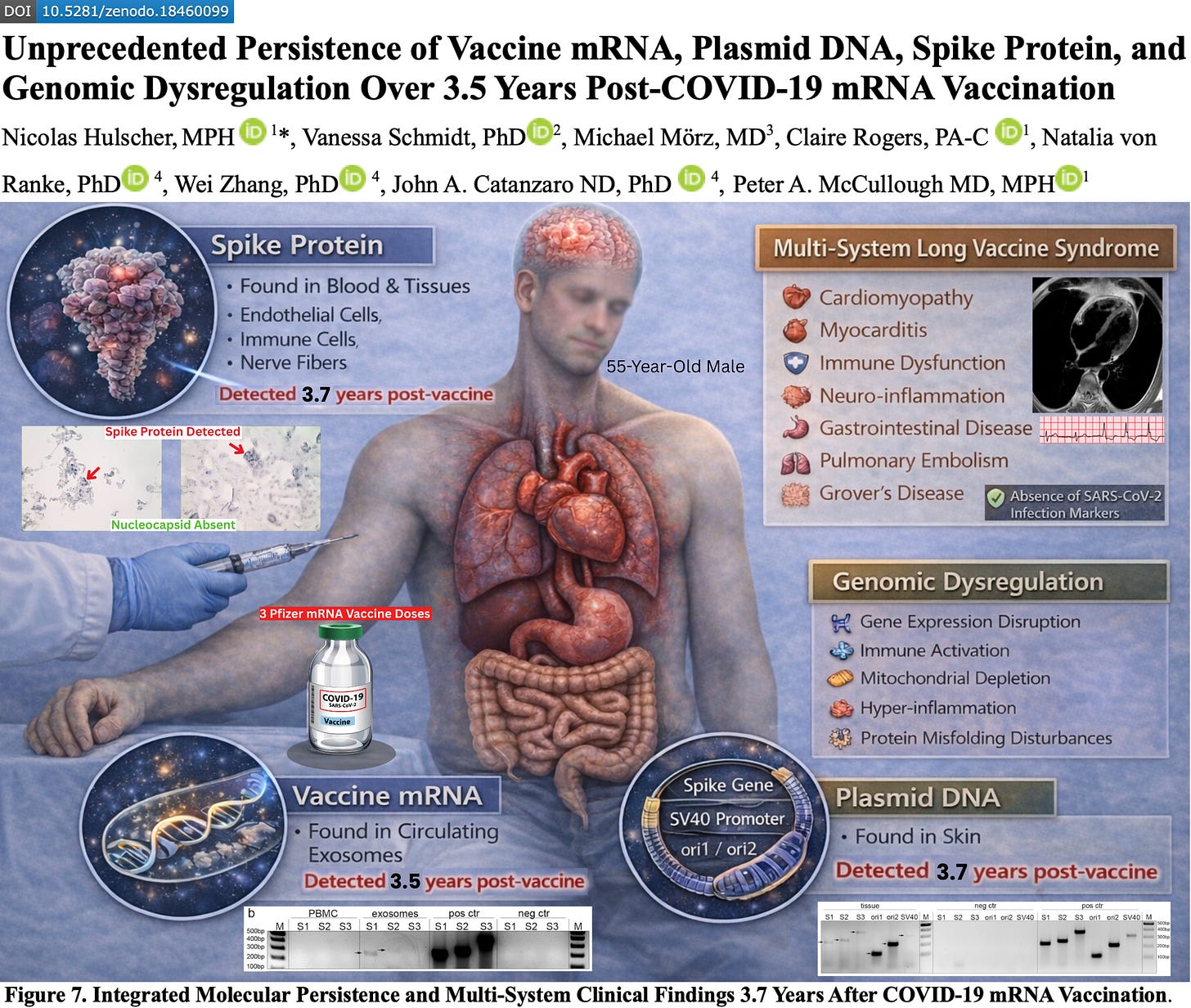
The resulting paper, titled “Unprecedented Persistence of Vaccine mRNA, Plasmid DNA, Spike Protein, and Genomic Dysregulation Over 3.5 Years Post–COVID-19 mRNA Vaccination,” presents what is, to our knowledge, the most comprehensive COVID-19 vaccine injury case report to date — involving >40 emergency department visits, >200 specialist encounters across 18 medical disciplines, >100 laboratory investigations, >100 imaging studies, and serial blood and tissue sampling performed at multiple timepoints over more than 3.5 years.
The findings reveal longitudinal molecular evidence that vaccine-derived mRNA, plasmid DNA fragments, and spike protein can persist in human blood and tissue more than 3.5 years after vaccination — independently confirmed across multiple laboratories using diverse analytical methods.
SARS-CoV-2 infection was effectively excluded: nucleocapsid antibodies remained negative across five separate timepoints and three independent laboratories, and nucleocapsid protein was absent in tissue specimens despite the presence of spike protein deposition.
Case Presentation
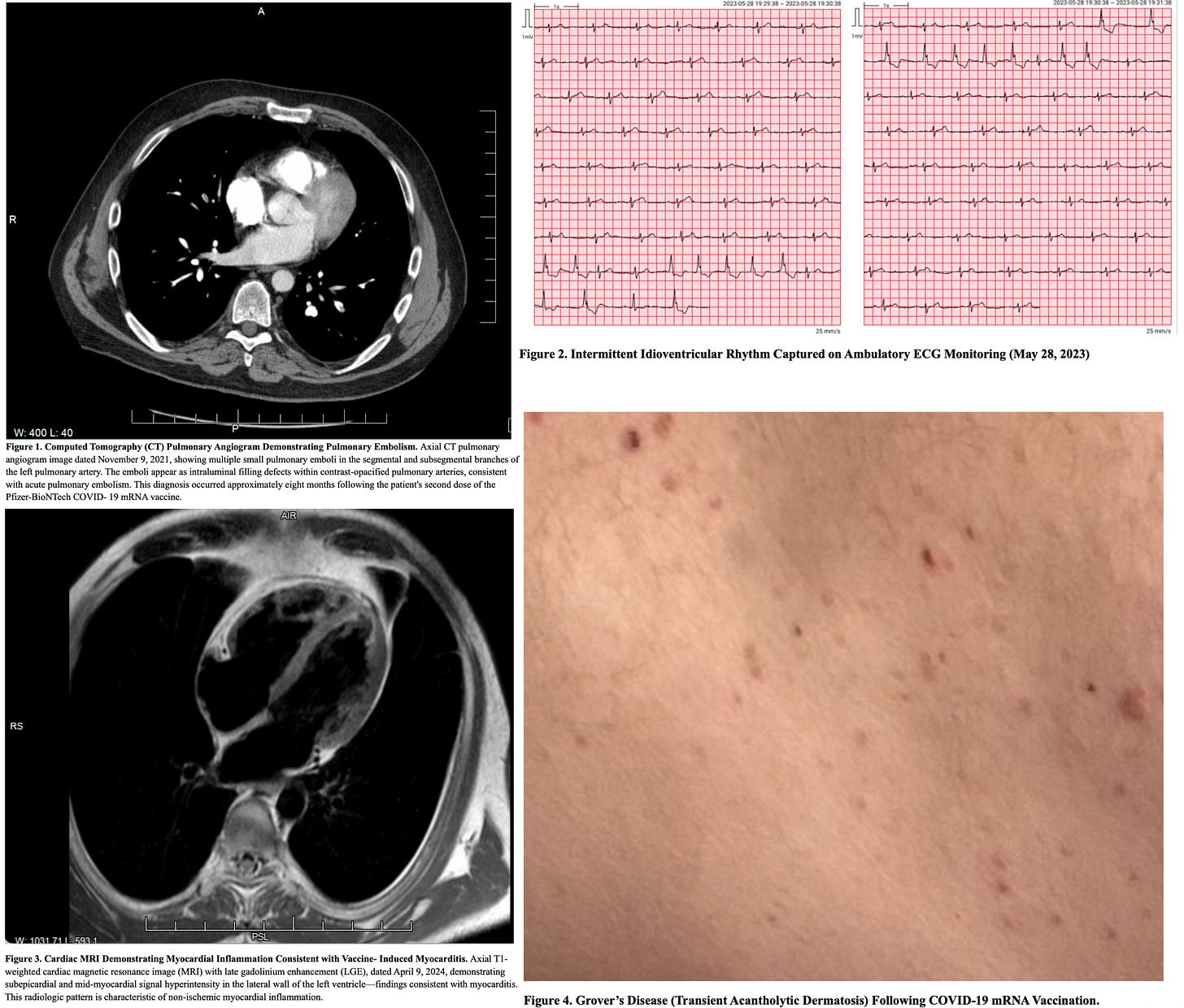
We report a 55-year-old male who received three doses of the Pfizer–BioNTech COVID-19 mRNA vaccine and subsequently developed progressive multi-organ dysfunction consistent with post-COVID-19 vaccine syndrome (PCVS), involving cardiopulmonary, neurologic, musculoskeletal, gastrointestinal, autonomic, otolaryngologic, audiovestibular, immune, ophthalmic, dermatologic, and psychiatric domains. Clinical manifestations included: pulmonary emboli; delayed MRI-confirmed myocarditis; neurocognitive impairment; small fiber neuropathy; autonomic dysfunction; myalgia; chronic pancreatic and gastrointestinal involvement; worsened tinnitus with sensorineural hearing loss; voice dysphagia and dysphonia; ophthalmic disturbances; chronic dermatologic inflammation; and anxiety/depression. The case was evaluated through a uniquely extensive longitudinal, multi-domain clinical investigation spanning molecular, immunologic, genetic, proteomic, transcriptomic, and tissue-based analyses, undertaken to characterize disease mechanisms and exclude alternative etiologies.
Extensive Diagnostic Evaluation
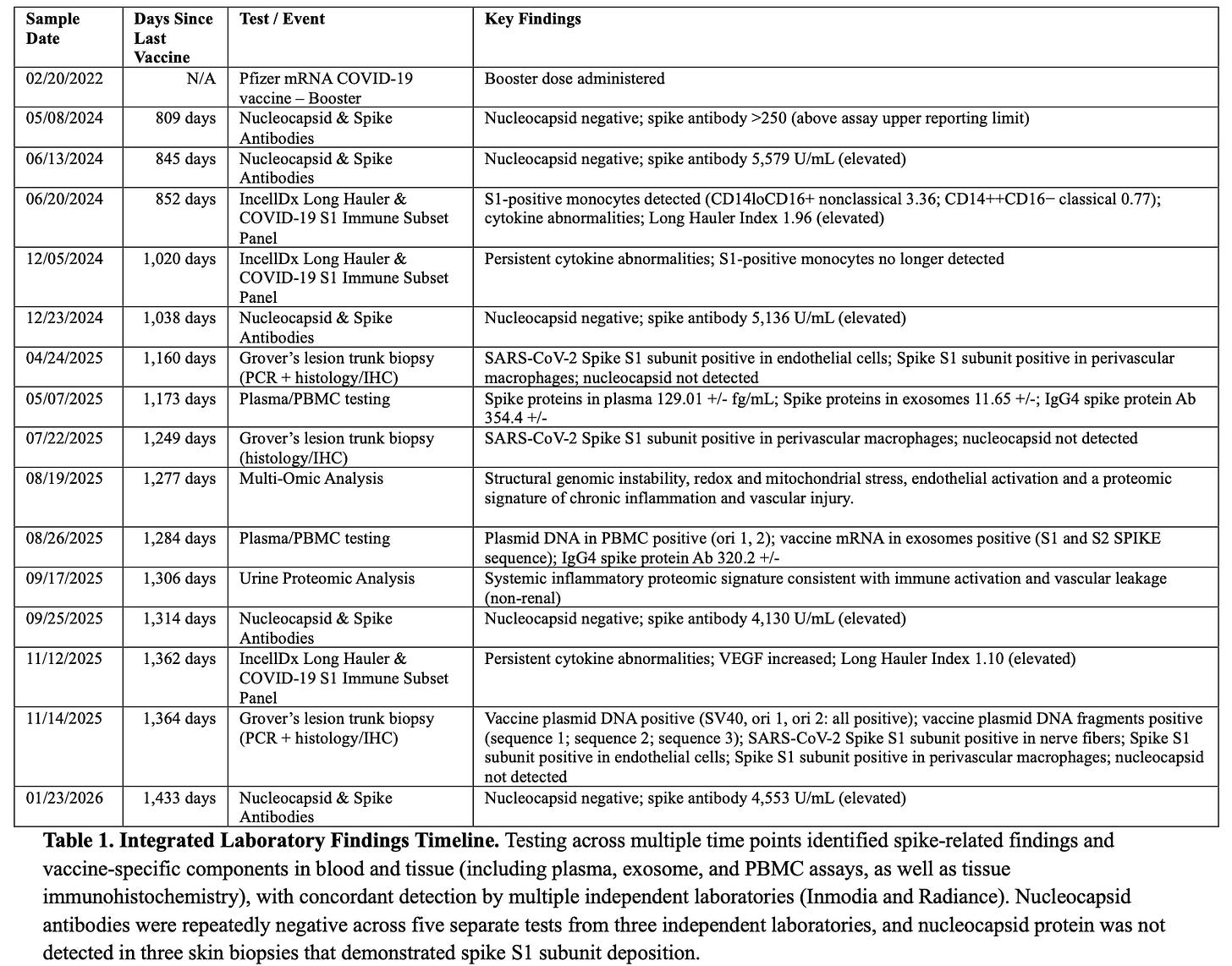
After >40 emergency department visits and >200 outpatient specialty encounters, the patient underwent >100 non-routine laboratory investigations and >100 imaging/functional studies. This evaluation systematically excluded underlying etiologic mechanisms across infectious, autoimmune, rheumatologic, endocrine, genetic, hematologic, malignant, toxic/medication-related, cardiovascular/vascular, metabolic, and primary neurologic domains. Testing remained largely nondiagnostic. A possible undocumented/undiagnosed asymptomatic infection manifesting as Long COVID was suspected after myocarditis diagnosis, and serology was pursued; unexpected results prompted expanded immune and tissue-based testing for spike- and vaccine-derived components. SARS-CoV-2 nucleocapsid antibodies were negative across five separate time points spanning 809–1,433 days post-vaccination, confirmed by three independent laboratories. The patient remains nucleocapsid negative with persistently elevated spike antibody levels (4,553 U/mL) 1,433 days after the final vaccination.
Specimen Collection and Analytical Methods
Blood and skin tissue specimens were obtained at multiple time points between 852–1,364 days after the final Pfizer–BioNTech COVID-19 mRNA vaccination. Biological compartments analyzed included plasma, circulating exosomes, peripheral blood mononuclear cells (PBMCs), and skin tissue. Specimens were evaluated across multiple independent laboratories using diverse analytical methodologies, including ELISA, automated immunohistochemistry, RT-PCR, standard PCR with Sanger sequencing confirmation, whole-genome sequencing, transcriptomic profiling, and quantitative mass spectrometry.
Persistent Circulating Spike Protein and Vaccine-Derived mRNA
At 852 days post-vaccination, blood-based immune testing identified detectable SARS-CoV-2 S1 protein within classical and non-classical monocyte subsets with associated cytokine and immune marker abnormalities.
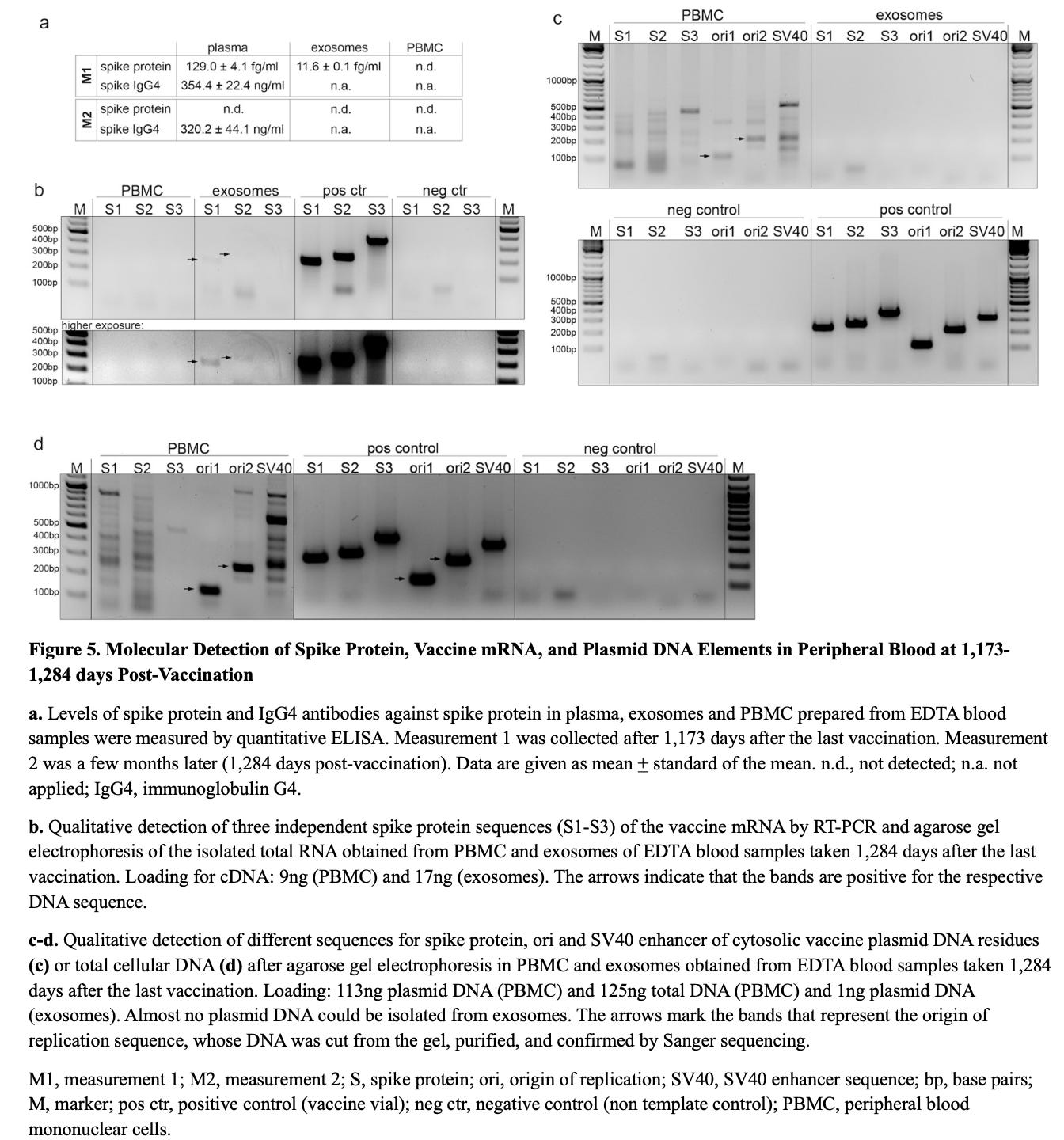
At 1,173 days post-vaccination, high-sensitivity ELISA detected free Wuhan spike protein in plasma (129.0 ± 4.1 fg/mL) and in circulating exosomes (11.6 ± 0.1 fg/mL).
At 1,284 days, RT-PCR identified vaccine-derived spike mRNA within circulating exosomes, whereas PBMC RNA remained negative following DNase-treated extraction and amplicon-specific PCR targeting three spike ORF regions (S1–S3).
Serologic profiling at 1,173 and 1,284 days post-vaccination demonstrated persistently elevated spike-specific IgG4 concentrations (354.4 ± 22.4 ng/mL and 320.2 ± 4.4 ng/mL, respectively), consistent with ongoing antigenic stimulation and an immune-tolerance–skewed response.
Persistent Spike Protein and Plasmid DNA in Skin Tissue
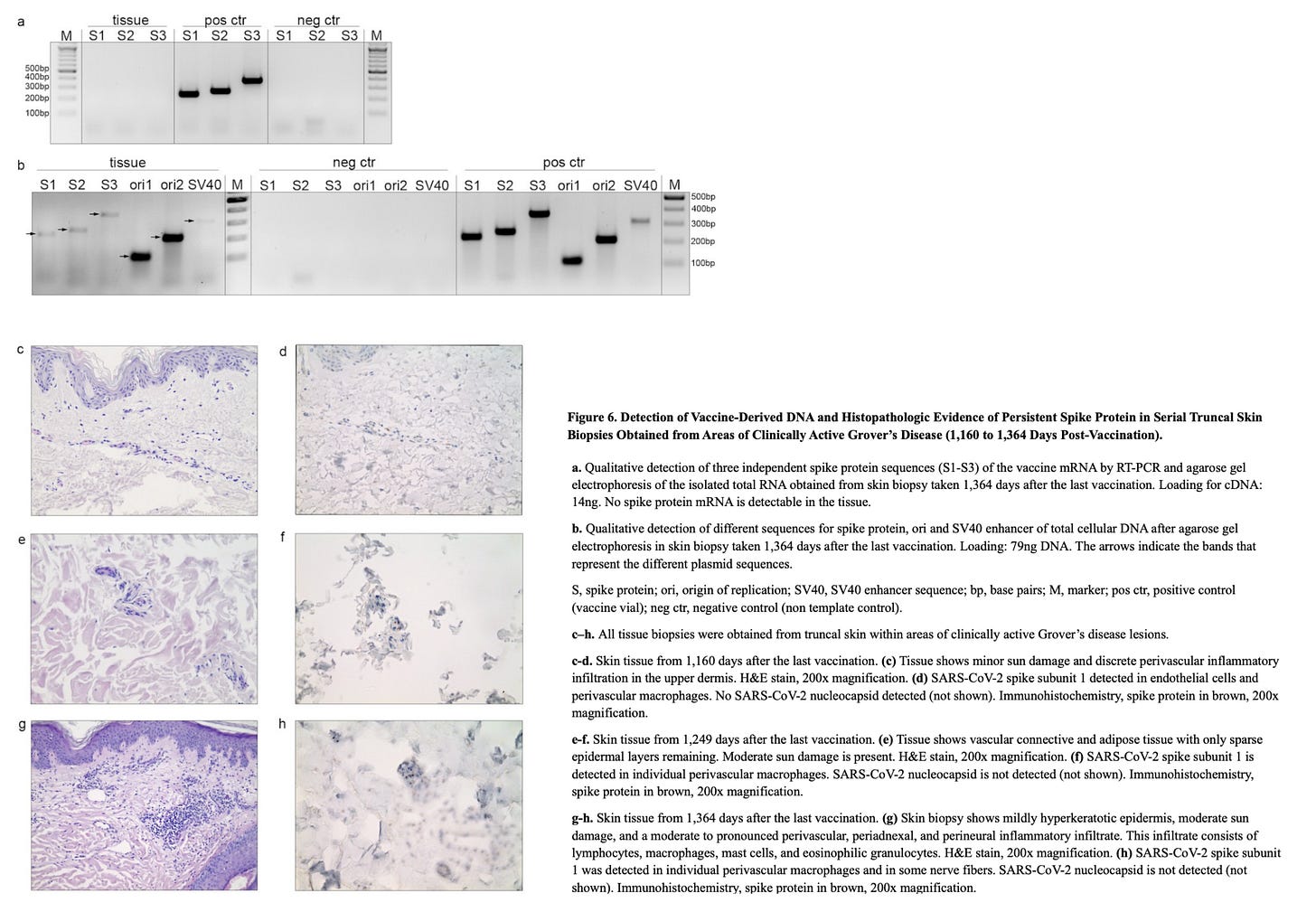
Serial skin biopsies at 1,160, 1,249, and 1,364 days post-vaccination, all from truncal skin within areas of clinically active Grover’s disease, were nucleocapsid negative and demonstrated persistent spike protein deposition in endothelial cells and macrophages by automated immunohistochemistry with histopathologic correlation. Spike protein was also found in nerve fibers at 1,364 days.
The 1,364-day skin biopsy contained multiple plasmid DNA elements, including spike gene sequences (S1–S3), ori1/ori2, and the SV40 enhancer, confirming durable retention of vaccine-derived DNA in somatic ti
Multi-Omic Analysis
Whole-genome sequencing structural variant analysis at 1,277 days post-vaccination revealed widespread genomic instability, with large duplications and deletions affecting EGFR, MYC, ERBB2, and ETV6/RUNX1, while RNA–DNA comparison showed RNA-only variants in ribosomal, NMD, small-RNA, epigenetic, and TP53 pathways.
Transcriptomic profiling of whole blood highlighted oxidative stress, vascular activation, and nuclear fragility.
Urine proteomics using quantitative mass spectrometry confirmed systemic inflammation with complement overactivation (CFH), redox imbalance (PRDX1), and sustained antibody responses, supported by risk alleles HLA-B07:02 and DRB1*11:04.
Conclusion
This case documents the longest reported in vivo persistence of vaccine-derived mRNA, plasmid DNA fragments, and spike protein following mRNA vaccination, with reproducible detection across multiple independent laboratories, distinct biological compartments, and complementary molecular detection systems extending beyond 3.5 years after the final dose. Spike protein, spike mRNA sequences, and plasmid backbone elements were identified in both immune cells and somatic tissue, with continued absence of SARS-CoV-2 nucleocapsid protein or antibodies, effectively excluding prior infection as the source. The convergence of these observations across longitudinal blood and tissue sampling provides direct evidence that mRNA vaccine-derived genetic material and its translated protein products can persist in vivo for years following administration.
In parallel, multi-omic analyses revealed sustained genomic instability and transcriptomic dysregulation more than 3.5 years post-vaccination, suggesting that persistent vaccine-derived material may be associated with long-term alterations in host genomic and molecular pathways.
These data challenge prevailing assumptions regarding rapid degradation and short-lived biological activity of mRNA vaccine components and underscore the need for controlled longitudinal studies to determine prevalence, mechanisms, and clinical consequences of persistent vaccine-derived material.
Epidemiologist and Foundation Administrator, McCullough Foundation
www.mcculloughfnd.org
Please consider following both the McCullough Foundation and my personal account on X (formerly Twitter) for further content.
Copyright 2026 Focal Points


What will be done about the DNA contamination in the COVID mRNA jabs?